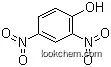
51-28-5Relevant articles and documents
Synthesis of 2,4-dinitrophenol
Khabarov, Yu.G.,Lakhmanov,Kosyakov,Ul'yanovskii
, p. 1577 - 1580 (2012)
New highly selective method is suggested for synthesis of 2,4-dinitrophenol by nitration of phenol with nitric acid in an aqueous-alcoholic medium at the boiling point of the reaction mixture. The yield of 2,4-dinitrophenol is as high as 80%. Pleiades Publishing, Ltd., 2012.
Metallohydrolase biomimetics with catalytic and structural flexibility
Mendes, Luisa L.,Englert, Daniel,Fernandes, Christiane,Gahan, Lawrence R.,Schenk, Gerhard,Horn, Adolfo
, p. 18510 - 18521 (2016)
The structural and functional properties of zinc(ii) complexes of two nitrogen rich polydentate ligands, HTPDP = 1,3-bis(bis-pyridin-2-ylmethylamino)propan-2-ol and HTPPNOL = N,N,N′-tris-(2-pyridylmethyl)-1,3-diaminopropan-2-ol, are compared. HTPDP is a hepta-dentate ligand with four pyridyl groups attached to a 1,3-diaminopropan-2-ol backbone while HTPPNOL contains only three pyridyl groups. In reactions with Zn(ClO4)2, HTPDP forms a dinuclear zinc compound [Zn2(TPDP)(OAc)](ClO4)2, 1. On the other hand, mononuclear [Zn(HTPPNOL)](ClO4)2, 2, and tetranuclear [Zn4(TPPNOL)2(OAc)3](ClO4)3, 3, complexes were isolated with the ligand HTPPNOL. Kinetic measurements with the substrate bis(2,4-dinitrophenyl)phosphate (BDNPP) revealed that compound 1 (kcat = 31.4 × 10?3 min?1) is more reactive than 3 (kcat = 7.7 × 10?3 min?1) at pH = 8.5, whilst the mononuclear compound 2 is inactive. Compound 1 displays a typical steady-state kinetic behaviour, while compound 3 exhibits steady-state behaviour only ~120 s after starting the reaction, preceded by a burst-phase. 31P NMR studies confirm that 1 can promote the hydrolysis of both ester bonds in BDNPP, generating the monoester DNPP and inorganic phosphate in the process. In contrast, DNPP is not a substrate for 3. The crystal structure of the complex formed by 3 and DNPP reveals the formation of a tetranuclear zinc complex [Zn4(TPPNOL)2(DNPP)2](ClO4)2, 4, in which the phosphate moiety of DNPP adopts an unusual tridentate μ-η1:η1:η1 coordination mode.
Functionalized graphene oxide as a nanocatalyst in dephosphorylation reactions: Pursuing artificial enzymes
Orth, Elisa S.,Fonsaca, Jéssica E.S.,Almeida, Thomas Golin,Domingues, Sergio H.,Ferreira, José G.L.,Zarbin, Aldo J.G.
, p. 9891 - 9894 (2014)
The present study reports for the first time the use of a thiol-functionalized graphene oxide nanocatalyst with impressive activity (>105-fold) in dephosphorylation reactions. The innovative and recyclable nanocatalyst has potential in designing artificial enzymes with targeted multifunctionalities and in detoxification of organophosphorus agents.
Sulfonatocalixarene Counterion Exchange Binding Model in Action: Metal-Ion Catalysis Through Host-Guest Complexation
Basílio, Nuno,Pessêgo, Márcia,Acu?a, Angel,García-Río, Luis
, p. 5397 - 5404 (2019)
p-Sulfonatocalixarene water soluble macrocyclic host receptors are known to form cooperative ternary complexes with complementary organic guest and metal cations. This property may be explored to enhance the interaction of weak nitrogen ligands with metal cations in a confined space showing some resemblance to metal-containing enzymes. However, the best of our knowledge, catalytic potential of this property remains unexplored. In this work the Ni2+ catalyzed hydrolysis of a picolinate ester (2,4-dinitrophenyl picolinate, 1) was used as a model reaction to evaluate the effect of sulfonatocalixarene macrocycles in the kinetics of this reaction. The results show that the host molecules promote the reaction through simultaneous complexation of the metal cation and the substrate and, in the case of the larger calixarenes containing more basic phenol groups, substantially higher rate enhancements are observed owing to additional assistance provided by base/nucleophilic catalysis. However, due the ionic nature of these receptors auto-inhibition of the reaction is observed at higher concentrations due counterion (Na+) binding that competes with the catalytically active Ni2+ -complexes.
Phosphodiester hydrolysis promoted by dinuclear iron(III) complexes
Piovezan, Clovis,Da Silva Lisboa, Fabio,Nunes, Fabio Souza,Drechsel, Sueli Maria
, p. 79 - 85 (2011)
We report the reactivity of three binuclear non-heme Fe(III) compounds, namely [Fe2(bbppnol)(μ-AcO)(H2O)2](ClO 4)2 (1), [Fe2(bbppnol)(μ-AcO) 2](PF6) (2), and [Fe2(bbppnol)(μ-OH)(Cl) 2]?6H2O (3), where H3bbppnol = N,N′-bis(2-hydroxybenzyl)-N,N′-bis(2-methylpyridyl)-1, 3-propanediamine-2-ol, toward the hydrolysis of bis-(2,4-dinitrophenyl)phosphate as models for phosphoesterase activity. The synthesis and characterization of the new complexes 1 and 3 was also described. The reactivity differences observed for these complexes show that the accessibility of the substrate to the reaction site is one of the key steps that determinate the hydrolysis efficiency.
AMINES AS LEAVING GROUPS IN NUCLEOPHILIC AROMATIC SUBSTITUTION REACTIONS. III. HYDROLYSIS OF 1-AMINO-2,4-DINITROBENZENES
Vargas, Elba Bujan de,Remedi, M. Virginia,Rossi, Rita de
, p. 113 - 120 (1995)
The kinetic study of the reaction of 1-pyrrolidino-2,4-dinitrobenzene, 1-pipridino-2,4-dinitrobenzene and 1-morpholino-2,4-dinitrobenzene with NaOH in the presence and absence of the amine leaving group was carried out in aqueous solutions at 25 deg C, giving 2,4-dinitrophenol as the only product.A mechanism involving the formation of ? complexes by addition of HO- or the amine to the unsubstituted positions of the aromatic ring is proposed.These complexes were found to react faster than the original substrates.
A Heterodinuclear FeIIIZnII Complex as a Mimic for Purple Acid Phosphatase with Site-Specific ZnII Binding
Roberts, Asha E.,Schenk, Gerhard,Gahan, Lawrence R.
, p. 3076 - 3086 (2015)
Purple acid phosphatases (PAPs) are the only dinuclear metallohydrolases for which the necessity for a heterovalent active site (FeIII-MII; M = Fe, Zn or Mn) for catalysis has been established. A major goal for the synthesis of PAP biomimetics is to design a ligand in which the two coordination sites exhibit discrimination between the trivalent and divalent metal ions. With this goal in mind the ligand 2-{[bis(2-methoxyethyl)amino]methyl}-6-{[(2-hydroxybenzyl)(2-pyridylmethyl)amino]methyl}-4-methylphenol (BMMHPH2), with two distinct coordination sites, N2O2 (α) and NO3 (β), has been prepared. Although not exactly mimicking the active site of PAP, the ligand facilitated the formation of the complex [FeIIIZnII(BMMHP)(CH3COO)2](BPh4), which exhibited regioselectivity in the two metal binding sites. The phosphoesterase-like activity of the complex in 50:50 acetonitrile/water was investigated by using the substrate bis(2,4-dinitrophenyl) phosphate (BDNPP) yielding kinetically relevant pKa values of 6.89, 7.37 and 9.00, a KM of 10.8±2.1 mM and a kcat of 3.20±0.38×10-3 s-1 (at pH = 7.5). Attempts to prepare a diiron analogue resulted in a centrosymmetric dimer, [FeIII2(BMMHPH)2(μ-OH)2](BPh4)2, with one six-coordinate FeIII atom in each of the α-sites, connected by two μ-hydroxido groups. In this Fe(μ-OH)2Fe diamond core the FeIII ions are weakly antiferromagnetically coupled, with J = -1.76±0.03 cm-1. The β-sites were vacant. Attempts to replace the ZnII ion with MgII resulted in the formation of a centrosymmetric trimer, i.e. [FeIII2MgII(BMMHPH)2(CH3COO)2(CH3O)2](BPh4)2.
An Approach to More Accurate Model Systems for Purple Acid Phosphatases (PAPs)
Bernhardt, Paul V.,Bosch, Simone,Comba, Peter,Gahan, Lawrence R.,Hanson, Graeme R.,Mereacre, Valeriu,Noble, Christopher J.,Powell, Annie K.,Schenk, Gerhard,Wadepohl, Hubert
, p. 7249 - 7263 (2015)
The active site of mammalian purple acid phosphatases (PAPs) have a dinuclear iron site in two accessible oxidation states (FeIII2 and FeIIIFeII), and the heterovalent is the active form, involved in the regulation of phosphate and phosphorylated metabolite levels in a wide range of organisms. Therefore, two sites with different coordination geometries to stabilize the heterovalent active form and, in addition, with hydrogen bond donors to enable the fixation of the substrate and release of the product, are believed to be required for catalytically competent model systems. Two ligands and their dinuclear iron complexes have been studied in detail. The solid-state structures and properties, studied by X-ray crystallography, magnetism, and M?ssbauer spectroscopy, and the solution structural and electronic properties, investigated by mass spectrometry, electronic, nuclear magnetic resonance (NMR), electron paramagnetic resonance (EPR), and M?ssbauer spectroscopies and electrochemistry, are discussed in detail in order to understand the structures and relative stabilities in solution. In particular, with one of the ligands, a heterovalent FeIIIFeII species has been produced by chemical oxidation of the FeII2 precursor. The phosphatase reactivities of the complexes, in particular, also of the heterovalent complex, are reported. These studies include pH-dependent as well as substrate concentration dependent studies, leading to pH profiles, catalytic efficiencies and turnover numbers, and indicate that the heterovalent diiron complex discussed here is an accurate PAP model system.
Kinetics of the pH-Independent Hydrolysis of Bis(2,4-dinitrophenyl) Carbonate in Acetonitrile-Water Mixtures: Effects of the Structure of the Solvent
El Seoud, Omar A.,El Seoud, Monica I.,Farah, Joao P.S.
, p. 5928 - 5933 (1997)
The pH-independent hydrolysis of bis(2,4-dinitrophenyl) carbonate, DNPC, in aqueous acetonitrile was studied spectrophotometrically from 20 to 45 °C. The binary solvent composition covers [H2O] from 0.02 to 51.39 M, corresponding to the water mole fraction, χw, from 0.100 to 0.971. The dependence of log (kobs), the observed rate constant, on χw is sigmoidal and is similar to the dependence of the solvent polarity scale ET(30) on χw for the same solvent mixture. As a function of decreasing χw, the Gibbs free energy of activation gradually increases, but ΔH? and ΔS? show a complex, quasi-mirror image dependence on χw. Plots of log (kobs) versus log [water] do not allow calculation of a single kinetic order with respect to water over the entire range of [water]. The structure of the transition state was probed by a proton inventory study carried out at χw = 0.453, 0.783, and 0.871, respectively. Plots of observed rate constants versus the atom fraction of deuterium in the solvent curve downward, and the results were fitted to a transition-state model that contains DNPC and two water molecules. Thus, the sigmoidal dependence of log (kobs) on log [water] is not due to an increase in the number of water molecules in the transition state as a function of increasing [water]. The similarity of plots of log (kobs) versus χw and ET(30) versus χw suggest similar solute- solvent interaction mechanisms, namely H-bonding and dipolar interactions. Kinetic results are discussed in terms of effects of the structure of acetonitrile-water mixtures on the solvation of reactant and transition states.
Kinetic Study of the Aminolysis and Pyridinolysis of O-Phenyl and O-Ethyl O-(2,4-Dinitrophenyl) Thiocarbonates. A Remarkable Leaving Group Effect
Castro, Enrique A.,Cubillos, Maria,Aliaga, Margarita,Evangelisti, Sandra,Santos, Jose G.
, p. 2411 - 2416 (2004)
The reactions of a series of secondary alicyclic (SA) amines with O-phenyl and O-ethyl O-(2,4-dinitrophenyl) thiocarbonates (1 and 2, respectively) and of a series of pyridines with the former substrate are subjected to a kinetic investigation in water, at 25.0 °C, ionic strength 0.2 M (KCl). Under amine excess over the substrate, all the reactions obey pseudo-first-order kinetics and are first-order in amine. The Broensted-type plots are biphasic, with slopes (at high pKa) of β1 = 0.20 for the reactions of SA amines with 1 and 2 and β1 = 0.10 for the pyridinolysis of 1 and with slopes (at low pKa) of β2 = 0.80 for the reactions of SA amines with 1 and 2 and β2 = 1.0 for the pyridinolysis of 1. The pKa values at the curvature center (pK a0) are 7.7, 7.0, and 7.0, respectively. These results are consistent with the existence of a zwitterionic tetrahedral intermediate (T?) and a change in the rate-determining step with the variation of amine basicity. The larger pKa0 value for the pyridinolysis of 1 compared to that for 2 (pKa0 = 6.8) and the larger pKa0 value for the reactions of SA amines with 1 relative to 2 are explained by the greater inductive electron withdrawal of PhO compared to EtO. The larger pKa0 values for the reactions of SA amines with 1 and 2, relative to their corresponding pyridinolysis, are attributed to the greater nucleofugalities of SA amines compared to isobasic pyridines. The smaller pKa0 value for the reactions of SA amines with 2 than with O-ethyl S-(2,4-dinitrophenyl) dithiocarbonate (pKa0 = 9.2) is explained by the greater nucleofugality from T? of 2,4-dinitrophenoxide (DNPO-) relative to the thio derivative. The stepwise reactions of SA amines with 1 and 2, in contrast to the concerted mechanisms for the reactions of the same amines with the corresponding carbonates, is attributed to stabilization of T? by the change of O- to S-. The simple mechanism for the SA aminolysis of 2 (only one tetrahedral intermediate, T?) is in contrast to the more complex mechanism (two tetrahedral intermediates, T? and T-, the latter formed by deprotonation of T? by the amine) for the same aminolysis of the analogous thionocarbonate with 4-nitrophenoxide (NPO-) as nucleofuge. To our knowledge, this is the first example of a remarkable change in the decomposition path of a tetrahedral intermediate T? by replacement of NPO- with DNPO- as the leaving group of the substrate. This is explained by (i) the greater leaving ability from T? of DNPO- than NPO- and (ii) the similar rates of deprotonation of both T? (formed with DNPO and NPO).
 This product is a nationally controlled contraband or patented product, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband or patented product, and the Lookchem platform doesn't provide relevant sales information.